LESSON PLAN
Name: Saba Waheed
Title of lesson: Properties of water
Date of lesson:
Length of lesson: 1-2 class periods.
Description of the class:
Name
of course: Chemistry
Grade
level: 9-12
Honors
or regular:
Source of the lesson:
The
following websites were used in this lesson:
- http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/H/HydrogenBonds.html
- http://www.exploratorium.edu/ronh/bubbles/sticky_water.html
- http://tidepool.st.usm.edu/crswr/hydrogenbond.html
- http://www.visionlearning.com/library/flash_viewer.php?oid=1380&mid=57
- http://www.visionlearning.com/library/flash_viewer.php?oid=1381&mid=57
TEKS addressed:
(1) Scientific processes. The student, for
at least 40% of instructional time, conducts field and laboratory
investigations using safe, environmentally appropriate, and ethical practices.
The student is expected to:
(A) Demonstrate
safe practices during field and laboratory investigations;
(2) Scientific
processes. The student uses scientific methods during field and laboratory
investigations. The student is expected to:
(A) plan
and implement investigative procedures including asking questions, formulating
testable hypotheses, and selecting equipment and technology;
(B) collect
data and make measurements with precision;
I.
Overview
The unique properties of
water are due to its structure and composition. Water molecule has two hydrogen
atoms with one oxygen atom. Each Hydrogen atom makes one covalent bond with
oxygen in such a way that they have an angular shape. This shape of the water
molecule makes it polar due to the partial negative charge on the oxygen and partial
positive charge on the hydrogen atoms. These partial charges allow water to
make hydrogen bonds with other water molecules, which gives water its unique
physical properties. Some of the unique physical properties of water due to
hydrogen bonding include high heat capacity, high specific heat, high boiling
point temperature, high surface tension, and cohesion-adhesion forces. This
concept is important for the students because the students will be able to
relate the use of water in their daily life to its properties.
II. Performance or
learner outcomes
Students
will be able to:
Ÿ Draw the structure of water and label the partial charges on oxygen and hydrogen.
Ÿ List the properties of water i.e. Density, Polarity, Solubility, Boiling point, cohesion, adhesion, and thermal properties.
Ÿ Define each property and give at least one example.
Ÿ Apply these properties to their daily lives through activities.
III. Resources, materials and supplies needed
¯ Projector.
¯ Transparencies of the notes (attached at the end of
lesson).
¯ Computer to show the simulations for the hydrogen
bonding.
IV. Supplementary materials, handouts.
o
Notes hand out.
o
Construction paper for
the evaluation part of lesson.
Five-E Organization
Teacher
Does Probing
Questions Student
Does
|
Engage: (5-8 mins) Students will answer questions as a
pre-assessment. (I may do this as a formal pre-assessment or as
a class discussion or group discussion). |
Students will answer the
following questions individually: 1. What is water made of? Draw the structure of water
molecule. 2. Which is denser? liquid water or ice? 3. How
does water stop the earth from getting too cold or too hot? 4. Write three things you learned about water from the
lab. |
Expected Student Responses/Misconceptions The students will be
expected to answer the questions as a participation grade. This will give me an
opportunity to observe some of the studentÕs misconceptions. |
|
Explore: (10-15mins) mins) Learning Experience(s) The students will
now be divided into groups of three. Students will relate the
lab activities to the properties of water. (Lab activities handout is
attached at the end of lesson plan) |
The students will be allowed to
use their books and computers as a resource to explore the reason behind the
behavior of water in each of the activities they did in lab. They will be asked to
write at least one reason for each activity and relate to the property of
water. |
Expected Student Responses/Misconceptions Students will explore the
different properties of water. |
|
Explain: (15-20) Learning Experience(s) The purpose of each activity will now be explained.
The students will be informed how each activity is related to the different
properties of water. |
I will explain the reason
for the water to be unique i.e. the concept of hydrogen bonding. I will show two simulations
from the following websites to help the students understand the concept of
hydrogen bonding: 1. http://www.visionlearning.com/library/flash_viewer.php?oid=1380&mid=57 2. http://www.visionlearning.com/library/flash_viewer.php?oid=1381&mid=57 I will give the students
the formal names for the properties of property and define each of the
following property of water.
(The definitions for each
of the above mentioned concept are on the lecture outline which is attached
at the end of lesson). |
Expected Student Responses/Misconceptions The students will take
notes and listen to the explanation for the activities they just did. |
|
Extend / Elaborate: Learning Experience(s) The students will apply the properties of water to its use
in cars which is a part of their project. They will be allowed to
use the resources such computer and library to find out how water is used in
cars? |
The students will assigned
to answer the following questions:
* Any questions they come
up will be discussed or answered individually* |
Expected Student Responses/Misconceptions The students will use
resources and answer the questions. This will help me to see how far the
students can relate the water properties to its use in cars. |
|
Evaluate: Students will be asked to make a concept map for the
properties of water. *Each student will make
their own concept map* |
A sample of concept map
will be displayed on the board to give them an understanding of how to make a
concept map. |
Expected Student Responses/Misconceptions Students will make the
concept make and relate the different concepts to each other and to their
daily lives. |
Percent effort each team member contributed to
this lesson plan:
100% Name of group member Saba Waheed.
¯ NOTES
FOR LECTURE:
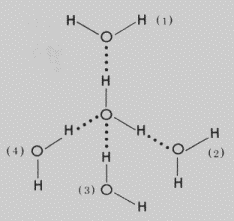
HYDROGEN BONDING:
A
relatively weak bond formed between a hydrogen atom and oxygen with an unshared
electron pair.
Molecules in water and other
liquids that undergo hydrogen bonding clump together. More energy is needed to
separate these particles so they have higher boiling points, heat capacity and
heat of vaporization than other liquids. Water has its unique physical
properties due to hydrogen bonding.
EXAMPLES:
POLARITY:
When the Hydrogen and Oxygen combine by the
sharing of electrons, the sharing is not equal, resulting in water being polar.
Consider what happens when an adult shares a candy bar with a child. Usually the adult, being bigger, gets a bigger portion of the candy bar. So it is with water.
Oxygen, being about 32 times more massive than Hydrogen, tends to pull the shared electrons closer to it, and does not share equally with the Hydrogen. Since the Oxygen has most of the electron, it also has more of a negative charge. The Hydrogen, having a smaller portion of the electrons, loses its negativity and becomes slightly positively charged. This type of bonding causes the water molecule to be polar, or charged at its ends.
BOILING POINT:
THE TEMPERATURE AT WHICH THE VAPOR PRESSURE OF A
LIQUID IS JUST EQUAL TO THE EXTERNAL PRESSURE ON THE LIQUID.
The boiling point of water is = 100¡C = 373.15 K
THE BOILING POINT OF
WATER IS HIGHER THAN MANY OTHER LIQUIDS SUCH AS ALCOHOLS AND VINEGAR.
SOLUBILITY.
THE SOLUBILITY OF A SUBSTANCE IS THE AMOUNT OF SOLUTE
DISSOLVED IN A GIVEN QUANTITY OF A SOLVENT.
More substances dissolve in water than in any other liquid. For this reason, water is often called the "Universal Solvent."
EXAMPLES:
- Water
and oil are not soluble.
- Table salt can dissolve in water because water molecules can surround the positive sodium ion or the negative chloride ion, the common components of table salt.
SURFACE TENSION
Surface
tension is caused by the attraction between the molecules
EXAMPLES:
Water's
high surface tension allows for the
á
formation of water droplets
and waves
á
allows plants to move water
(and dissolved nutrients) from their roots to their leaves
á
The movement of blood through tiny vessels in the bodies of
some animals.
á
It is due to the high
surface tension of water that insects can walk on the water surface.
ADHESION
THE QUALITY OR CONDITION OF STICKING TOGETHER OR HOLDING FAST.
Adhesion is attraction of water molecules to other substances.
For example:
Adhesion is seen:
When water is dropped on tissue paper, it climbs up to the tissue fibres.
COHESION
The attraction
of one water molecule to
another resulting from hydrogen
bonding.
The ability of water molecules to quickly break and re-form hydrogen bonds gives it a property called cohesion.
EXAMPLES:
- This is why water falls from the sky as raindrops, and not
individual molecules,
- Water tends to bead up on the hood of your freshly waxed car
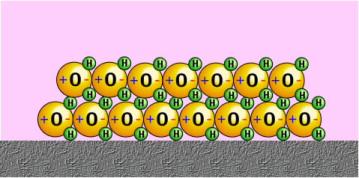
DENSITY
The density of a substance (liquid, solid, or gas) is defined as the mass of that substance per unit volume.

If an object is less dense than water than it will float, and if it is denser than water it will tend to sink.
EXAMPLES:
Ice floats because it is less dense than water.
Oil is observed to float on water because it is less dense than water.
Same applies to floating logs, and ducks.
If ice sank, it is very likely that ice skating would never have been invented and fishes in the lakes and rivers would not be able to survive.
.